1️⃣ Why Paris is Europe's Robotics Compliance Hub
#DigitalSovereignty #FrenchTech #AIRegulation
Paris has emerged as Europe's leading robotics and AI hub, driven by France's €2.5 billion National AI Strategy targeting 2030 leadership. But with opportunity comes regulatory responsibility.
2025 Game-Changer: France launched INESIA (National Institute for the Evaluation and Safety of AI), giving French companies a critical local compliance partner alongside the EU AI Office.
Key for Manufacturers: While the EU AI Office centralizes power (causing "legal confusion" about the AI Board's role), INESIA ensures Paris-based firms have localized safety evaluation and faster guidance on robotics-specific challenges.
🔴 New Criminal Liability: France's Law No. 2024-449 (Article 226-8-1) now criminalizes non-consensual AI-generated deepfakes—critical for firms developing humanoid robots or advanced computer vision systems.
2️⃣ The Timeline: Critical Compliance Deadlines
#AIDeadlines #RegulatoryCompliance #ISO42001
The EU AI Act (entered force August 1, 2024) has a tiered transition schedule that most companies misunderstand. Here's what you need to know:
| Date | Regulatory Milestone | What It Means |
|---|---|---|
| Feb 1, 2025 | Prohibited AI Banned | ✅ ACTIVE NOW - Emotion recognition in workplaces, social scoring, biometric categorization—must be decommissioned |
| Aug 1, 2025 | GPAI Rules Active | ✅ ACTIVE NOW - General Purpose AI models (like LLMs) must comply with Chapter V transparency rules |
| Aug 1, 2026 | ⚠️ MAIN DEADLINE | 6 MONTHS AWAY - High-risk systems, transparency requirements, AIMS frameworks mandatory |
| Aug 1, 2027 | Extended Transition | Medical devices, machinery under Annex I harmonization legislation |
⏰ You Have 6 Months to implement full compliance for high-risk systems. Documentation requirements are extensive—if you haven't started, you're already behind.
💡 Beyond Compliance: The Competitive Advantage
Even if the August 2026 deadline is extended (unlikely, but possible), AI trust and operational clarity are already major selling points for stakeholders:
- Investors demand proof of responsible AI governance before funding
- Enterprise clients require vendor compliance certifications in RFPs
- Insurance providers are pricing AI liability based on governance maturity
- Board members need transparency to manage fiduciary risk
- Customers increasingly choose transparent AI over "black box" competitors
Early compliance isn't just risk mitigation—it's a trust signal that differentiates you in the market.
3️⃣ Your 6-Point Compliance Roadmap
#ComplianceChecklist #AIGovernance #RiskManagement
Here's what robotics manufacturers MUST have in place by August 2026:
✅ 1. Risk Classification & Assessment
- • Determine if your system falls under Annex III (high-risk categories)
- • Document risk mitigation measures
- • Critical infrastructure, employment, biometric systems = automatic high-risk
✅ 2. Technical Documentation (Annex IV)
- • Training data specifications and validation datasets
- • Complete system architecture diagrams
- • Design specifications and safety protocols
- • This is your primary evidence for regulatory audits
✅ 3. Post-Market Monitoring (PMM) Plan
- • Critical deadline: Feb 1, 2026 - EU standardized PMM templates due
- • Establish continuous monitoring systems NOW if not already done
- • Track system performance, incidents, and safety metrics
✅ 4. Supply Chain Management (Article 25)
- • Adopt voluntary model contractual terms for suppliers
- • Ensure third-party components meet transparency standards
- • Document entire value chain from components to final assembly
✅ 5. AI Management System (AIMS)
- • ISO 42001 framework provides the blueprint
- • Required for ongoing safety and governance
- • Note: Article 63 allows simplified AIMS for microenterprises (but transparency requirements remain high)
✅ 6. Incident Reporting System (Article 73)
- • Serious incidents must be reported within strict timelines
- • Automated monitoring recommended
- • Healthcare AI has additional reporting requirements under MDR
4️⃣ Medical Robotics: The Dual Compliance Challenge
#MedicalDevices #HealthTech #AIinHealthcare
French medical robotics companies face a complex dual-regulatory environment: EU AI Act + Medical Devices Regulation (MDR) 2017/745.
Why This Matters:
- 🏥 France's 2025 Health AI Roadmap confirms AI as a pillar of national healthcare strategy
- ⚖️ Updated Product Liability Directive lowers threshold for consumer claims against defective AI medical devices—strict compliance = financial necessity
- 📋 2021 Bioethics Law integrated AI into French Public Health Code, creating permanent legal obligations for healthcare robotics
Smart Strategy: Leverage CNIL's "sandbox" for AI regulatory guidance. CNIL has evolved from data protection advisor to AI "soft law" leader, essential for navigating CCNE/CNPEN guidance on AI diagnostics.
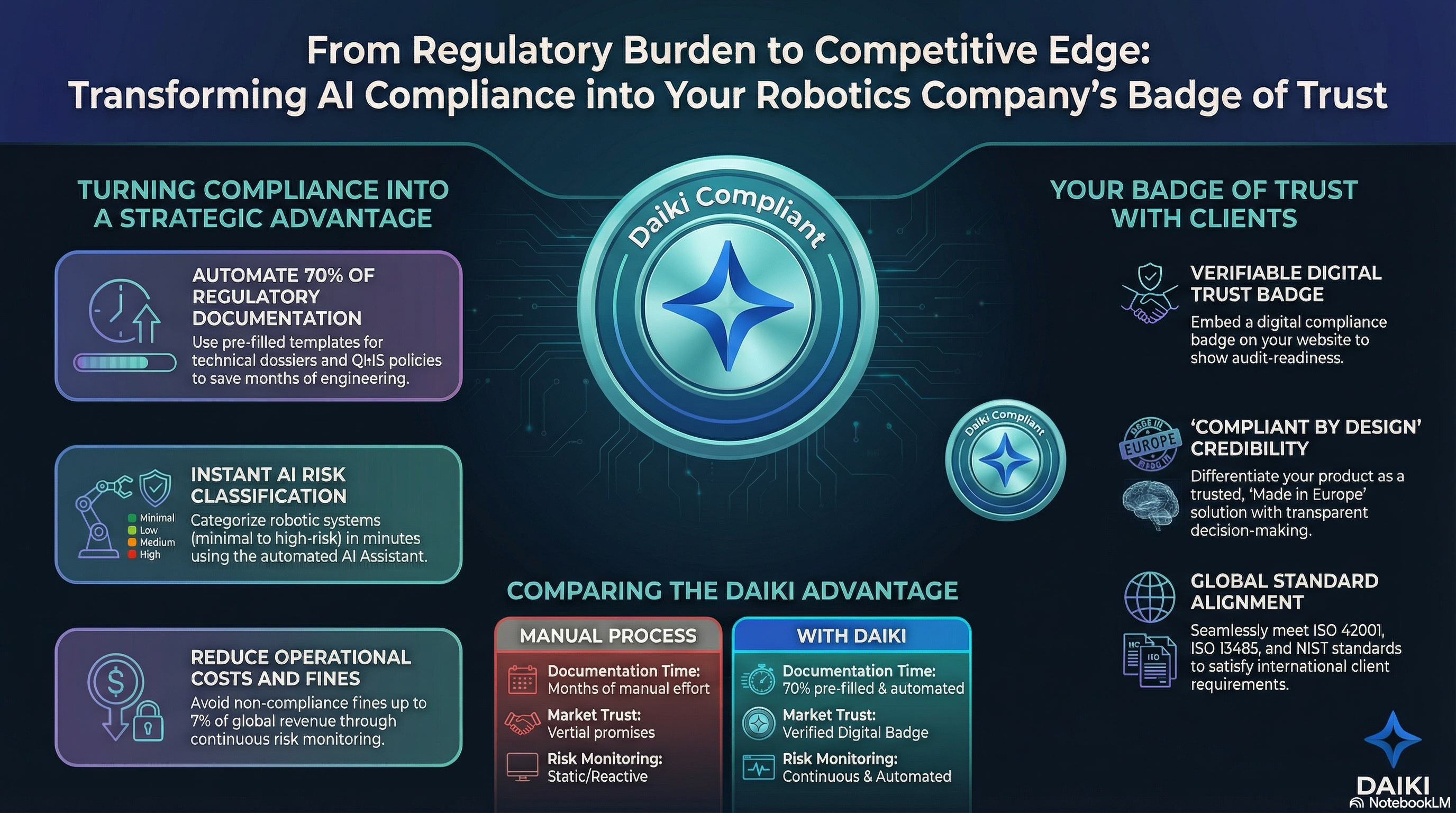
5️⃣ How Daiki Simplifies Compliance
#DaikiPlatform #ComplianceSoftware #AITools
The EU AI Act's administrative burden requires more than legal counsel—you need integrated technical solutions.
Daiki specializes in:
🔹 AIMS Implementation (Article 63)
Tailored quality management systems for medical robotics microenterprises—full safety without crushing administrative weight
🔹 Automated Post-Market Monitoring
Ready for the Feb 1, 2026 PMM template deadline with automated data collection and reporting architecture
🔹 Incident Reporting (Article 73)
Specialized monitoring ensuring serious incidents meet strict timelines for both AI Act and healthcare safety mandates
🔹 Annex IV Documentation
Streamlined technical documentation generation and maintenance
All built and hosted in Europe. Full GDPR compliance. Zero data retention.
6️⃣ France vs. Global AI Regulation Landscape
#GlobalCompliance #AIRegulation #RegulatoryComparison
For Paris firms with international operations, here's how France compares:
| Jurisdiction | Approach | Key Deadlines |
|---|---|---|
| 🇫🇷 France (EU) | Comprehensive risk-based via EU AI Act; INESIA + EU AI Office oversight | Aug 1, 2026 (general); Aug 1, 2027 (Annex I) |
| 🇺🇸 United States | Sectoral/state-led; rollback of federal AI orders (Jan 2025); state algorithmic discrimination laws | Feb 2026 (Colorado); Jan 1, 2026 (California) |
| 🇬🇧 United Kingdom | Non-statutory/sectoral; AI Opportunities Action Plan + "AI Airlock" sandbox approach | No fixed deadline; ongoing sectoral guidance |
Insight: EU's comprehensive approach creates short-term compliance burden but long-term competitive advantage through regulatory clarity and global standard-setting.
🚨 5 Critical Mistakes to Avoid
#ComplianceMistakes #RiskMitigation #BestPractices
-
❌ Waiting until July 2026 to start technical documentation
✅ Start NOW—you're already 6 months from deadline with extensive Annex IV requirements
-
❌ Treating PMM as an afterthought
✅ Build monitoring systems into your product architecture from day one
-
❌ Ignoring Article 25 supply chain requirements
✅ Document your entire value chain and third-party components
-
❌ Assuming GDPR compliance = AI Act compliance
✅ They overlap but have distinct requirements—don't conflate them
-
❌ Not leveraging CNIL sandbox for healthcare AI
✅ Free regulatory guidance that prevents costly mistakes
🎯 Ready to Prepare for August 2026?
#GetCompliant #FreeAudit #AIConsulting
The countdown to August 2026 represents a fundamental transition to "Trustworthy AI"—but the regulatory path is complex. With only 6 months remaining, the window for comprehensive preparation is closing fast.
Even if deadlines shift, the business case is clear: AI trust and operational clarity are no longer optional—they're competitive necessities. Stakeholders demand it, clients require it, and your market position depends on it.
From Law No. 2023-451 on transparency to the shifting competences of the EU AI Office, failure to align with these mandates by 2026 and 2027 deadlines will result in significant market barriers and potential penalties of up to €35 million or 7% of global revenue.
🆓 Start with a Free Flash Audit
Get your EU AI Act compliance readiness score in 10 minutes:
- ✅ Instant 0-100 compliance score
- ✅ Gap analysis of critical requirements
- ✅ Priority action roadmap
- ✅ Personalized recommendations
💼 Or Book a Strategic Consultation
Discuss how to align your robotics projects with August 2026 mandates and learn about our specialized Daiki-powered compliance services:
Book a Call →